Swallow-Breath Interaction and Phase of Respiration with Swallow During Nonnutritive Suck in Term Infants and Preterm Infants Approaching Term Adjusted Age
Eric W. Reynolds1*, Cynthia S. Bell2, Debbie Grider3
1University of Texas Health Science Center at Houston, McGovern Medical School, Department of Pediatrics, Division of Neonatology Houston, TX
2University of Texas Health Science Center at Houston, McGovern Medical School, Department of Pediatrics, Houston, TX
3University of Kentucky, Department of Pediatrics, Division of Neonatology, Lexington, KY
Abstract
Introduction: During nonnutritive suck, infants must intermittently swallow. When a swallow occurs, it must interact with respiration in 2 main ways. We have previously labeled HOW the interaction occurs as “swallow-breath interaction” (SwBr), and WHERE in the respiratory cycle the swallow occurs as “phase of respiration incident to swallow” (POR). We have described SwBr and POR in preterm infants with and without bronchopulmonary dysplasia and term infants with neonatal abstinence syndrome.
Objective: The objective of this work is to describe SwBr and POR in term infants (TRM) and compare those findings to our previous study of low-risk preterm (LRP) infants.
Method: Suckle, swallow, nasal airflow and chest movement were recorded during nonnutritive suck in 12 TRM infants, collecting 94 swallows. SwBr and POR for each swallow were characterized by our previously described method. Generalized estimating equations were developed to relate the 3 types of SwBr and 5 types of POR to gender, birth weight, gestational age, postmenstrual age (PMA), and weeks post-first nipple feed. The percentages of SwBr and POR were compared to 16 LRP infants, with 176 swallows over 35 encounters.
Results: TRM infants had more swallows with attenuated respiration (AR) with advancing weeks post-first nipple feed and fewer swallows occurring with obstructive apnea (OA) in males and with increasing birth weight. More swallows occurred at mid-expiration (ME) with increasing gestational age, PMA, and male gender and at mid-inspiration (MI) with increasing weeks post-first nipple feed. Fewer swallows occurred at MI in males. Infants in the LRP group studied before 35 weeks PMA were different from TRM infants but become indistinguishable from TRM infants as PMA approached 40 weeks. SwBr and POR in LRP infants progress towards improved feeding efficiency and safety. These results are similar to studies of nutritive feeding.
Conclusion: SwBr and POR during nonnutritive suck in LRP infants become more like TRM infants with advancing PMA. Because the same brainstem centers are activated in both nutritive and nonnutritive suck, investigation of swallow during nonnutritive suck may provide similar information as nutritive feeding with easier analysis.
Introduction
Efficient suckle feeding can be considered to be the most complex skill a newborn infant must master to attain independent survival. However, feeding problems are frequent in preterm infants1 and can lead to prolonged hospital stays2. Poor feeding in the neonatal period may be an early indicator of neurologic injury3,4 and has been linked to language delay later in life5. Abnormal suck during nutritive feeding has been associated with abnormal verbal, performance and total IQ at primary school age6. Thus, the development of suck-swallow-breath rhythms during newborn feeding may be an early marker of neurologic development.
The development of efficient suckle-feeding is dependent on the maturation and coordination of neuronal central pattern generators (CPGs) controlling suck, swallow and breath7. These same CPGs are activated, to varying degrees, during nonnutritive suck since swallows still occur, although much less frequently than during nutritive feeding. Thus, nonnutritive suck may provide an earlier marker of neurodevelopment than nutritive feeding.
During nonnutritive suck, suckle occurs regularly and often, but swallow occurs infrequently since the infants need only swallow when they have collected enough of their own oral secretions, or fluid leakage from the study catheters to necessitate a swallow. When a swallow does occur, the swallow and breathing must abruptly interact for 1-2 seconds, in what has been termed “deglutition apnea,” and should not be confused with apnea of prematurity which requires 15-20 seconds of cessation of breathing to be defined as apnea.
When a swallow does occur, we can identify 3 types of swallow-breath interaction (SwBr): central apnea (CA) [cessation of both nasal airflow and chest movement], obstructive apnea (OA) [cessation of nasal airflow but continued rhythmic chest movement], or attenuated respiration (AR) [a slight deflection of the slope of the respiratory line on the graph at the time of the swallow without disruption of the respiratory rhythm]. The respiratory cycle can be divided into the following phases (POR): Beginning Expiration (BE), Mid-Expiration (ME), End-Expiration (EE), Mid-Inspiration (MI) and Apnea (AP). We have previously published figures showing examples of each type of SwBr and POR8. This is similar to the swallow-respiratory interfacings described by Amaizu, et al9.
We have previously used our method to study nonnutritive suck in low-risk preterm (LRP) infants8 and have shown that SwBr and POR develop in predictable patterns in these infants. Our results supported the fact that the progression of SwBr in LRP infants is influenced by increasing opportunities to practice the skill, or what can be considered “learning”. The progression of POR in these infants was more affected by measures of maturation, indicating a developmental progression. We have also described SwBr and POR in preterm infants affected by bronchopulmonary dysplasia10 and term infants with neonatal abstinence syndrome11. Our method has not yet been applied to healthy term infants.
The objective of this study is to describe SwBr and POR in term infants (TRM) during nonnutritive suck and compare those results to our findings from low-risk preterm infants.
Methods
This is a descriptive study of patients enrolled in our larger study of nonnutritive suck in infants affected by various health conditions. The study participants included 12 healthy TRM infants and a group of 16 LRP infants. The TRM infants included babies born between 37 and 42 weeks of gestation, appropriate size for gestational age, 5-minute Apgar score of 7 or more and with no congenital anomalies or metabolic disorders who were born at our hospital and admitted to the normal newborn area of the hospital. A total of 94 swallow events occurred over 12 encounters from these infants. The LRP group included 16 infants who were born at less than 36 0/7 weeks of gestation, appropriate size for gestational age, no congenital anomalies, no intraventricular hemorrhage of grade 3 or 4 and deemed to be ‘low-risk’ for bronchopulmonary dysplasia (BPD) per the definition developed for our previous study describing SwBr and POR in this group8,10. Infants were studied once per week from the onset of oral feeding until discharge from the NICU. There were 176 swallow events collected from 35 encounters in this group. TRM and LRP infants were enrolled prospectively during the same time period.
To evaluate the effect of maturation, the entire LRP group (LRP-All) was divided into 3 subgoups by postmenstrual age (PMA) at the time of the study. LRP-Early includes encounters that occurred before a PMA of 35 weeks and included 98 swallows among 19 encounters. LRP-Mid includes encounters that occurred when PMA was between 35 0/7 weeks and 39 0/7 weeks and contained 59 swallows among 12 encounters. LRP-Late are encounters that occurred when PMA was >39 weeks and consisted of 19 swallows among 4 encounters. The number of LRP-Late encounters is limited because most preterm infants in this type of low-risk category are discharged from the NICU prior to reaching this age. There were 5 swallows in the LRP group for which POR could not be determined due to limitations in the data set (2 in LRP-Early, 3 in LRP-Mid). These swallows were not used in the analysis of POR.
Informed consent was obtained from the parent(s) of each infant prior to the infant’s participation in the study. The project complies with all applicable HIPAA standards and was approved by the Institutional Review Board of the University of Kentucky.
We have previously published the specific method for preparing the babies for the study and data collection8. To summarize the salient portion of the study for this project, the study participants were prepared in the following manner:
• A 5F nasopharyngeal catheter was placed and connected to a pressure transducer (Transpac IV Neonatal/Pediatric Pressure Monitoring Kit, Hospira Inc., Lake Forrest IL) to measure swallow pressure. The nasopharyngeal catheter was placed by measuring the distance from the nose to the lower portion of the ear and then to the angle of the mandible.
• A second catheter was placed through the back of pacifier so that the catheter tip was flush with the bulb of the nipple and connected to a transducer to measure suckle pressure.
• Respiratory effort was measured with a strain gauge attached to a stretchable band placed around the infant’s chest (Pneumotrace II, Model 1132, UFI, Morro Bay, CA).
• Nasal airflow was measured with a small thermistor bead (Omega 44030, Omega Engineering Inc., Stamford, CT) in a custom assembly placed at the opening of the nares.
• Other biometric data (ECG, SpO2), 2 types of acoustic data, and short- and long-term developmental outcomes were collected for use in other studies.
This is the same method used for our study of preterm infants with bronchopulmonary dysplasia and term infants with neonatal abstinence syndrome10-11. Figure One is a diagram of the instrumentation used on each study infant.
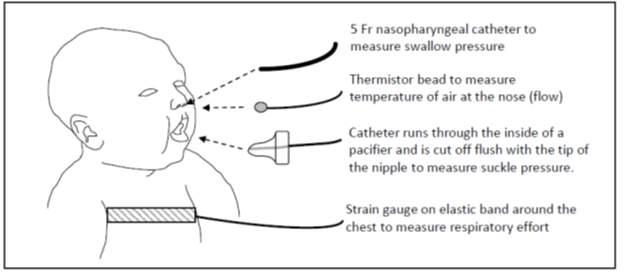
Figure 1: Diagram of instrumentation of a study infant.
In general, infants tolerated the instrumentation well with a few infants experiencing some gagging or coughing as the nasopharyngeal catheter was placed. This was considered to be insignificant given the fact that many infants, particularly the LRP group, regularly have nasogastric tubes in place. With the equipment in place, the infant was offered a pacifier for one minute of NNS immediately prior to a regularly scheduled feeding time.
Data were displayed as multi-channel linear graphs, using the Windaq Acquisition System and Waveform Browser (Dataq Industries, Akron OH). The entire one-minute sequence of NNS was canvassed for swallow events, noted as deflections in the nasopharyngeal pressure recording. The type of SwBr was classified, as described below. The phase of respiration incident to swallow was also identified. Weekly encounters allowed us to look at changes in SwBr over time. Swallow categorization was performed by a single individual (EWR) who was blinded to the characteristics of each study except for group assignment. Thus, the reviewer knew that the study came from the LRP or TRM group, but did not know the gestational age, postmenstrual age, etc…associated with any individual study.
Dependent variables include the 3 types of SwBr and 5 types of POR identified for each swallowing event. Independent variables for this analysis included gender, birth weight, gestational age, postmenstrual age, and weeks post-first nipple feed (time between first nipple feed and day of study). Day of life at the time of the study is not included because it would create a collinearity issue in the data set as postmenstrual age is a function of gestational age and day of life. Weeks before-first nipple feed (the time from birth to first nipple feeding) was used in our analysis of LRP infants but is not used in the analysis of the TRM group. All babies in the TRM group began oral feeding on the day of birth, thus weeks before-first nipple feed for all TRM babies is zero.
We used SAS (Cary, NC) to calculate descriptive statistics for patient demographics and to construct logistic regression models relating the odds of each type of SwBr and POR to the independent variables defined above. Statistical inferences were made via generalized estimating equations12 with an exchangeable structure to take into account the correlations inherent to repeated assessments on the same baby. The percentage of each type of SwBr and POR were compared across the TRM and LRP groups with the Wilcoxon Rank Sums Test and Pairwise Two-Sided Multiple Comparison Analysis.
Results
Table One shows the demographic data describing the TRM and LRP groups. All studies in the TRM group occurred between the infants’ 2nd and 5th days of life. Data describing the LRP infants confirms that the group is typical of convalescing preterm infants and clearly different from the TRM group.
Table 1: Demographic Data for TRM and LRP Groups. P-value for comparisons of all variables between groups is <0.001
| TRM | Mean | Standard Deviation | Range |
|---|---|---|---|
| Gestational Age (weeks) | 39.5 | 0.95 | 37.6 - 40.9 |
| Male Gender | 5 (of 12) | ||
| Birth weight (gms) | 3344 | 398 | 2630 - 3930 |
| Day of Life (days) | 2.5 (median) | 2 - 5 | |
| Postmenstrual age (weeks) | 39.8 | 0.92 | 37.9 - 41 |
| Weeks Before-First Nipple Feed (weeks) | 0 | 0 | 0 |
| Weeks Post-First Nipple Feed (weeks) | 0.3 | 0.19 | 0.14 - 0.71 |
| LRP | Mean | Standard Deviation | Range |
| Gestational Age (weeks) | 28.7 | 2.4 | 24.6 - 35 |
| Male Gender | 9 (of 16) | ||
| Birth weight (gms) | 1056 | 338 | 520 - 1990 |
| Day of Life (days) | 50 (median) | 8 - 87 | |
| Postmenstrual age (weeks) | 35.4 | 2.4 | 32.1 -41.3 |
| Weeks Before-First Nipple Feed (weeks) | 4.5 | 1.9 | 0.3 - 8.6 |
| Weeks Post-First Nipple Feed (weeks) | 2.6 | 1.9 | 0 - 8.14 |
Table Two shows the descriptive statistics for the TRM group as related to the independent variables. There were actually 5 independent variables related to each type of SwBr and POR yielding 40 potential comparisons. We have only shown the 8 statistically significant relationships to simplify the data display. All other comparisons were not statistically significant. Term babies had more swallows at AR with advancing weeks post-first nipple feed and fewer swallows at OA with increasing birth weight. Male infants also had fewer swallows at OA than females. TRM group infants had more swallows at ME with increased gestational age, postmenstrual age, and male gender. They had slightly more swallows at MI with increased weeks post-first nipple feed. Male infants had fewer swallows at MI.
Table 2: Statistically Significant Relationships Between SwBr, POR and the Independent Variables: Generalized estimating equations were calculated relating each type of SwBr (AR, OA, CA) and POR (BE, ME, EE, MI, AP) to the independent variables (gestational age, postmenstrual age, birth weight, gender, weeks post-first nipple feed), giving 40 possible comparisons. Only the 8 statistically significant relationships are shown.
| SwBr | OR | p-value | |
|---|---|---|---|
| AR | Weeks Post-First Nipple Feed | 1.86 | 0.0379 |
| OA | Birth weight | 0.003 | 0.0411 |
| Male Gender | 0.3104 | 0.0371 | |
| POR | |||
| ME | Gestational Age | 2.2577 | 0.0091 |
| Postmenstrual Age | 2.4593 | 0.0063 | |
| Male Gender | 2.2577 | 0.0055 | |
| MI | Weeks Post-First Nipple Feed | 1.085 | 0.0377 |
| Male Gender | 0.1922 | 0.0242 |
Descriptive statistics for the LRP group have been previously published8. The results were interpreted to show that there is a progression of SwBr toward more swallows occurring with AR and fewer at CA and OA as these infants approach 40 weeks post-menstrual age. There is also a progression toward more swallows occurring at BE as these infants age. Furthermore, the progression of SwBr is influenced by opportunities to practice. The progression of POR was more affected by measures of maturation regardless of the time available to practice feeding.
Figure Two shows the distribution of SwBr in TRM and LRP infants and for the LRP encounters divided into 3 epochs by PMA (Early: <35 wks, Mid: 35-39 wks, Late: >39 wks). In TRM infants, most swallows occur at AR (74%). 20% occur at OA and 5% at CA. All 3 types of SwBr in the LRP-All group are statistically different from TRM infants. This is also true for the LRP-Early group. For LRP-Mid, only AR and CA are statistically different from TRM. The LRP-Late group is statistically indistinguishable from the TRM infants.
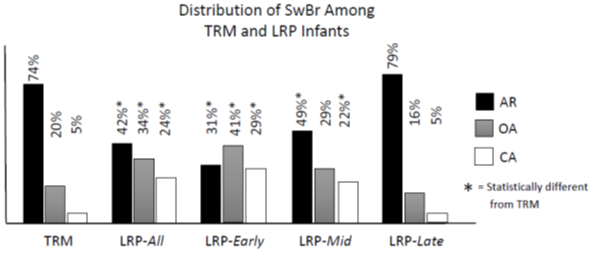
Figure 2: Distribution of SwBr for Each Group. TRM: Term, LRP-All: entire LRP group, LRP-Early: PMA<35wks, LRP-Mid: PMA 35 0/7 – 39.5 wks, LRP-Late: PMA>39.5wks. AR: Attenuated Respiration, OA: Obstructive Apnea, CA: Central Apnea. * = Statistically different from TRM
Figure Three shows the distribution of POR for TRM and LRP infants and LRP infants divided by PMA. For POR in TRM infants, most swallows occur at points in the respiratory cycle that appear to be protective against aspiration because airflow is absent or outward. This includes BE at 41%, ME at 19% and AP at 10%, for a combined 70% of swallows. There are 3 statistical differences found between LRP-All group and TRM infants (BE, ME and EE). For LRP-Early, only BE and EE are statistically different from TRM. There are no statistical differences between TRM and either LRP-Mid or -Late.
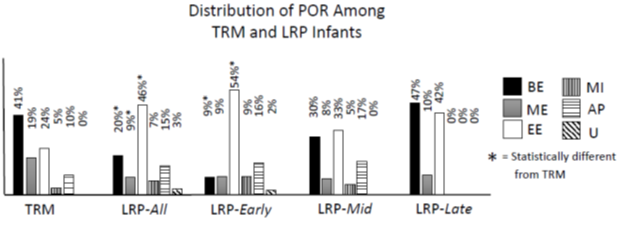
Figure 3: Distribution of POR for Each Group. TRM: Term, LRP-All: entire LRP group, LRP-Early: PMA<35wks, LRP-Mid: PMA 35 0/7 – 39.5 wks, LRP-Late: PMA>39.5wks. BE: Beginning Expiration, ME: Mid-Expiration, EE: End-Expiration, MI: Mid-Inspiration, AP: Apnea, U: Undeterminable.
Discussion
The study of newborn feeding can be considered to be an evaluation of the function of the neonatal brainstem, since the ability to rhythmically suckle feed is dependent on afferent stimuli, central integration and efferent function of brainstem reflexes13. Nonnutritive suck requires integrity of the same neurologic pathways as rhythmic feeding, even though swallow occurs comparatively infrequently when the infant must clear the pharynx of his/her own secretions. Since infants are capable of nonnutritive suck at an earlier age than nutritive feeding, it is reasonable to consider the coordination of suck, swallow and breath during nonnutritive suck to be an earlier indication of neurologic integrity than nutritive feeding.
Indeed, the evidence for a link between nonnutritive suck and nutritive feeding has long been established. Pickler et al, has shown nonnutritive suck to improve oxygen tension and behavioral state during feeding when it is offered just prior to a feeding14. The provision of nonnutritive suck decreases the transition time from gavage to full oral feeding, accelerates the maturation of the sucking reflex during feeding, decreases intestinal transit time and induces more rapid weight gain in infants, leading to a shorter length of stay for preterm infants15-17. Patterned orocutaneous therapy effectively accelerates non?nutritive suck development and oral feeding success in preterm infants who are at risk for oromotor dysfunction18.
We have previously used our method to describe the coordination of suck, swallow and breath rhythms during nonnutritive suck in low-risk preterm infants8, preterm infants affected by bronchopulmonary dysplasia10, and in infants affected by neonatal abstinence syndrome11. In our work with low-risk preterm infants, we have shown that SwBr and POR develop in predictable and measurable ways. Infants with neonatal abstinence syndrome have SwBr and POR characteristics more similar to low-risk preterm infants rather than term infants of equivalent gestational age. We now turn to evaluating SwBr and POR in healthy term babies.
We found relatively few significant relationships within the TRM group. This is not surprising, given the fact that the independent variables in this group are rather tightly packed. Specifically, all of the encounters in the TRM group took place over a 4-day of life period between the infants’ 2nd and 5th days of life and a postmenstrual age range of just over three weeks. Interestingly, even with these limitations, we still found a significant increase in AR associated with weeks post-first nipple feed. This is indicative of a very rapid progression toward more AR in the first days after the birth of a term infant. The association between AR and weeks post-first nipple feed in the LRP group, from our previous work, was also significant8. Thus, the frequency of opportunities to practice feeding has a meaningful effect on the progression of SwBr in both preterm and term infants, even in the first few days of life of a healthy term infant’s life.
When comparing the distribution of SwBr between the TRM and the whole LRP group we found all three types of SwBr to be significantly different between the two groups. The TRM group had more swallows occurring with AR and fewer swallows occurring at both OA and CA. The distribution of POR between the two groups also revealed significant differences. Specifically, TRM infants had more swallows at BE and ME and fewer occurring at EE. There were also fewer swallows in the TRM group occurring at MI and AP, but these differences were not statistically significant.
In TRM infants, the high percentage of swallows occurring at AR is likely a marker for the maturity of suck-swallow-breath integration as this type of SwBr allows for the continuation of suck, swallow and breath during rhythmic suckle feeding. The high percentage of POR occurring at BE, ME and AP is likely protective against aspiration. BE and ME are protective against aspiration as exhalation occurs after bolus passage, effectively clearing the airway of any residual fluid. AP is also protective against aspiration in that the lack of air movement in either direction prevents residual fluid from moving into the airway.
In our previous work with LRP infants, we found a progression of SwBr from CA and OA toward more AR with advancing weeks before-first nipple feed, weeks post-first nipple feed and gestational age and a similar progression for POR toward more swallows at BE8. We hypothesized at the time that this represented a maturational progression of what could be considered normal. This conjecture would be confirmed if it could be shown that the SwBr and POR characteristics in the LRP group were becoming more like healthy term infants. Thus, we divided the LRP encounters into three epochs based on the PMA at which the study occurred; Early (before 35 0/7 weeks), Mid (35 0/7 – 39 0/7 weeks) and Late (>39 0/7 weeks). When we compared the distributions of SwBr between LRP-Early and TRM infants, we found all three types of SwBr were statistically different between the two groups, with more TRM swallows occurring at AR and fewer swallows at OA and CA. The distribution of SwBr types in the LRP-Mid group is closer to that of TRM infants, but continues to be statistically different from TRM for AR and CA. There are no statistical differences between the LRP-Late and TRM infants. There is a similar, but somewhat weaker, progression of the distribution of POR types. Compared to TRM infants, the LRP-Early group shows statistical differences, and nonsignificant trends, in the percentage of each type of POR. Specifically, LRP-Early has fewer swallows at BE and ME and more at EE than TRM infants. However, no statistical differences are found between LRP-Late and TRM infants. Thus, the LRP infants are becoming more like healthy TRM infants over time in both SwBr and POR characteristics.
Interpretation of the results is weakened by the small number of encounters included in the older LRP groups. Most preterm infants, particularly these low-risk infants, are discharged from their initial NICU stay prior to reaching 39 weeks PMA. Despite this limitation, our results are consistent with studies of nutritive feeding. Other authors have found that swallows during nutritive feeding of preterm infants at lower postmenstrual ages occur predominantly with apneic swallow runs. With maturation, there are fewer apneic swallows19-24. Since rhythmic swallow is not required during nonnutritive suck, it is a commonly accepted view that nonnutritive suck is independent of the swallow and respiration. Hence, nonnutritive suck matures earlier and occurs at a higher frequency than suckle during nutritive feeding25. However, our findings suggest that even though swallows are occurring infrequently during nonnutritive suck, there is still a measurable progression of the swallow-breath characteristics (both SwBr and POR) as infants mature. Furthermore, low-risk preterm infants are becoming more like term infants as they mature or “learn” to coordinate suck, swallow and breath. This finding is consistent with studies of oral stimulation strategies of nonnutritive suck, or training of oromotor central pattern generators, that have shown to be helpful in the development of nutritive feeding26-30. We would also suggest that because there are fewer swallows over time with nonnutritive suck, it may be easier to study than nutritive feeding (it certainly is with our method), representing a possible advantage of the study of nonnutritive suck over nutritive feeding for this particular aspect of newborn development.
Conclusion
There is a rapid progression of swallow-breath interaction toward the mature AR form in the days after the birth of a term baby. Swallow-breath interaction during nonnutritive suck in low-risk preterm infants becomes more like that of term infants as the convalescing preterm infants approach 40 weeks PMA. Nonnutritive suck and nutritive feeding are generally considered separate processes due to the absence of sustained rhythmic swallows during nonnutritive suck. However, with this method our results with nonnutritive suck seem to parallel studies of nutritive feeding. Swallows during nonnutritive suck may be easier to study due to comparatively fewer swallows occurring during nonnutritive suck. We hypothesize that since the maturation of SwBr and POR seem to follow a similar progression in both nonnutritive suck and nutritive feeding, it is likely that interventions to improve the coordination of swallow during nonnutritive suck may be helpful for improving nutritive feeding. Further work in this area will include describing nutritive feeding in these groups, as well as nonnutritive suck and nutritive feeding in infants with various pathologies.
References
- Bennett FC. Neurodevelopmental outcome in low birth weight infants: The role of developmental intervention. In R. Guthrie (ed). Clinics in Critical Care Medicine. no13: Neonatal Intensive Care. New York: Churchill Livingstone. 1988; pp221-249.
- Vandenberg KA. Nippling management of the sick neonate in the NICU: The disorganized feeder. Neonatal Network. 1990; 9(1): 9-16.
- Ingram TTS. Clinical significance of the infantile feeding reflexes. Dev Med Child Neurol. 1962; 4: 159-169.
- Volpe JJ, Hill H. Disorders of sucking and swallowing in the newborn infant: Clinicopathological correlations. In R Korofiken, C Guilleminault (eds). Progress in Perinatal Neurology. Baltimore:Williams & Wilkins. 1981; Vol 1: pp.157-181.
- Adams-Chapman I, Bann CM, Vaucher YE, et al. Association between feeding difficulties and language delay in preterm infants using Bayley III. J Pediatr. 2013 Sept; 163(3): 680-685.e3.
- Wolthuis-Stigter M, Da Costa SP, Bos AF, et al. Sucking behaviour in infants born preterm and developmental outcomes at primary school age. Dev Med Child Neurol. 2017 Aug; 59(8): 871-877.
- Barlow SM. Oral and respiratory control for preterm feeding. Curr Opin Otolaryngol Head Neck Surg. 2009 Jun; 17(3): 179-186.
- Reynolds EW, Grider D, Caldwell R, et al. Swallow-breath interaction and phase of respiration with swallow during nonnutritive suck among low-risk preterm infants. Am J Perinatol. 2010 Nov; 27(10): 831-840.
- Amaizu N, Shulman RJ, Schanler RJ, et al. Maturation of oral feeding skills in preterm infants. Acta Paediatr. 2008 Jan; 97(1): 61-67.
- Reynolds EW, Grider D, Caldwell R, et al. Effects of Bronchopulmonary Dysplasia on Swallow:Breath Interaction and Phase of Respiration With Swallow During Nonnutritive Suck. J Nat Sci. 2018 Sept; 4(9): Pii e531.
- Reynolds EW, Grider D, Bell CS. Swallow-breath interaction and phase of respiration with swallow during nonnutritive suck in infants affected by neonatal abstinence syndrome. Front Pediatr. 2017; 5: 214.
- Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986; 73: 13–22.
- Nishino T. The swallowing reflex and its significance as an airway defensive reflex. Front Physio. 2013; 3: 489.
- Pickler RH, Frankel HB, Walsh KM, et al. Effects of nonnutritive sucking on behavioral organization and feeding performance in preterm infants. Nurs Res. 1996 May-June; 45(3): 132-135.
- Field T, Ignatoff E, Stringer S, et al. Nonnutritive sucking during tube feedings: Effect on preterm neonates in an intensive care unit. Pediatrics. 1982; 70: 381-384.
- Measel CP, Anderson GC. Nonnutritive sucking during tube feedings: Effect on clinical course in premature infants. JOGN Nurs. 1979 Sept-Oct: 8(5): 265-272.
- Bernbaum JC, Pereira GR, Watkins JB, et al. Nonnutritive sucking during gavage feeding enhances growth and maturation in premature infants. Pediatrics. 1983 Jan; 71(1): 41-45.
- Poore M, Zimmerman E, Barlow SM, et al. Patterned orocutaneous therapy improves sucking and oral feeding in preterm infants. Acta Paediatrica. 2008; 97(7): 920-927.
- Bamford O, Taciak V, Gewolb I. The relationship between rhythmic swallowing and breathing during suckle feeding in term neonates. Pediatr Res. 1992; 31: 619-624.
- Koenig JS, Davies AM, Thach BT. Coordination of breathing, sucking and swallowing during bottle feeding in human infants. J Appl Physiol. 1990; 69: 1623-1629.
- Mathew OP. Respiratory control during nipple feeding in preterm infants. Pediatr Pulmonol. 1988; 5: 220-224.
- Hanlon MB, Tripp JH, Ellis RE, et al. Deglutition apnoea as indicator of maturation of suckle feeding in bottle-fed preterm infants. Dev Med Child Neurol. 1997; 39: 534-542.
- Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr. 2003; 92: 721-727.
- Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning and coordination of respiration and swallow during feeding in preterm infants. Dev Med Child Neurol. 2006; 48: 589-594.
- Lau C. Development of infant oral feeding skills: what do we know? Am J Clin Nutr. 2016; 103(Suppl): 616S-621S.
- Barlow SM, Finan DS, Chu S, et al. Patterns for the premature brain: synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. J Perinatol. 2008; 28: 541-548.
- Poore M, Zimmerman E, Barlow SM, et al. Pattern orocutaneous therapy improves sucking and oral feeding in preterm infants. Acta Pediatr. 2008; 97(7): 920-927.
- Fucile S, Gisel EG, Lau C. Effect of an oral stimulation program on sucking skill maturation of preterm infants. Dev Med Child Neurol. 2005; 47(3): 158-162.
- Rocha AD, Moreira ME, Pimenta HP, et al. A randomized study of the efficacy of sensory-motor-oral stimulation and nonnutritive sucking in very low birth weight infant. Early Hum Dev. 2007; 83(6): 385-388.
- Fucile S, Gisel E, Lau C. Oral stimulation accelerates the transition from tube to oral feeding in preterm infants. J Pediatr. 2002; 141: 230-236.
APPENDIX
Explanation of Abbreviations
| TRM | Term Infants | Babies born between 37 and 42 weeks with no congenital anomalies and no signs or symptoms of illness. |
| LRP(-All) | Low-Risk Preterm | Group of 'healthy' preterm infants with no sepsis, no IVH and relative low-risk for the development of bronchopulmonary dysplasia |
| LRP-Early | Low-Risk Preterm Early | LRP infants studied before 35 0/7 weeks postmenstrual age |
| LRP-Mid | Low-Risk Preterm Mid | LRP infants studied between 35 0/7 and 39 0/7 weeks gestation |
| LRP-Late | Low-Risk Preterm Late | LRP infants studied after 39 0/7 weeks gestation |
| SwBr | Swallow-Breath Interaction | How swallow interacts with breath. Can occur in 3 types (AR, OA, CA). |
| AR | Attenuated Respiration | Deflection of the slope of the nasal airflow tracing without interruption in the overall breathing rhythm |
| OA | Obstructive Apnea | Cessation of nasal airflow for the duration of a swallow with continued chest movement |
| CA | Central Apnea | Cessation of both nasal airflow and chest movement for the duration of a swallow |
| POR | Phase of Respiration Incident to Swallow | Where in the respiratory cycle a swallow occurs. Can occur in 5 types (BE, ME, EE, MI, AP) |
| BE | Beginning Expiration | Transition from inspiration to expiration |
| ME | Mid-Expiration | Point between beginning expiration and end expiration |
| EE | End Expiration | Transition from expiration to inspiration |
| MI | Mid-Inspiration | Point between end expiration and beginning expiration |
| AP | Apnea | Period of no discernable breathing for 1 second prior to the time of a swallow |
