Pre-exposure Prophylaxis as HIV Prevention in High Risk Adolescents
1*Megan E. Gray, 2Sheela V Shenoi, 3Rebecca Dillingham
1*Division of Infectious Diseases and International Health. University of Virginia, Charlottesville, VA
2Assistant Professor of Medicine. Yale University, New Haven, CT
3Director of the Center for Global Health, Associate Professor of Medicine. Division of Infectious Diseases and International Health. University of Virginia, Charlottesville, VA
Introduction
A recently published review of the pediatric Human Immunodeficiency Virus (HIV) continuum of care in Pediatric Clinics of North America discussed methods of HIV prevention for children and adolescents1. HIV pre-exposure prophylaxis (PrEP), the use of antiretroviral medications in uninfected persons to prevent HIV infection, was mentioned in the review. The use of PrEP in high risk adolescent populations is an emerging practice. Significant knowledge gaps remain, but the available data to guide current practice is growing due to the vigorous state of PrEP research. A dedicated discussion of PrEP for select adolescents is merited.
Adolescence refers to the period of transition between childhood and adulthood, which begins with puberty and is characterized by psychological, cognitive, social, socioeconomic and sexual development. The exact ages that encompass adolescence are arguably indistinct, though the age range of 10-19 is used by the World Health Organization (WHO)2,3. Most of the research done to establish efficacy and safety of PrEP has been conducted with predominantly adult populations, or those over the age of 18. Few PrEP studies exclusively among adolescents have been done, though more studies are underway4-7.
The first study evaluating oral PrEP among men who have sex with men (MSM) was published in 2010 and showed efficacy of once daily tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC)8. Several studies followed9-11, all including patients >18yo, leading the United States Food and Drug Administration (FDA) to approve TDF-FTC (trade name Truvada®) for PrEP use in 2012. Labeling specifies that use is for adults at high risk for HIV, though clarification on what age is considered adulthood is not given12. While TDF-FTC is presently the only approved formulation of PrEP, several formulations have been studied, including intravaginal gels13,14, monthly intravaginal rings15, long acting intramuscular injections16, and long-acting subcutaneously implanted polymer devices17.
Consensus Guidelines
Currently, there are no countries that have approved the use of oral PrEP for adolescents4. However, several organizations have issued guidelines regarding PrEP in sexually active adolescent populations. The WHO revised their guidelines in 2015 to recommend PrEP to any person with substantial risk of HIV infection as a part of a comprehensive prevention bundle. This was a change from the WHO’s 2012 guidelines, which recommended PrEP only for MSM, transgender people, and sero-discordant couples. The WHO defines substantial risk as HIV incidence of greater than 3 per 100 person-years in the absence of PrEP usage2. This risk may be difficult to assess on an individual or sub-population basis. However, there are certain groups at elevated risk and country-dependent vulnerable populations that have substantial risk based on available epidemiologic data.
The United States Centers for Disease Control and Prevention (CDC) briefly discusses the use of PrEP in adolescent minor populations within their 2014 PrEP clinical practice guideline, recommending that the risks and benefits should be carefully considered in the framework of applicable laws and regulations as there is insufficient data on the use of PrEP in adolescent populations18. The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) developed their own PrEP guidelines in 2015, which recommended prioritizing PrEP for adolescent girls and young women (AGYW), ages 15-24, in the ten countries targeted by the PEPFAR DREAMS initiative19.
United Nations International Children’s Emergency Fund (UNICEF) and New York State Department of Health AIDS Institute both have published reports summarizing meetings that discuss challenges in and priorities for implementation of PrEP in adolescent populations20,21. Challenges and concerns raised by a panel of young advocates and youth taking PrEP at the UNICEF meeting are presented in Table 121.
Table 1: Challenges in PrEP Implementation for High Risk Adolescents Raised by a Youth Panel
| Challenges in PrEP Implementation for High Risk Adolescents |
|---|
| Legal constraints related to age of consent for sexual and reproductive health care |
| Sexual violence and non-supportive sexual partners |
| PrEP cost |
| Discrimination related to sexual orientation, gender, or addiction disorders |
| Stigma from health service providers, the public, and adolescents’ friends, family and sexual partners |
| Limited capacity to assess HIV acquisition risk |
PrEP in High Risk Groups and Vulnerable Populations
Adolescent Girls and Young Women
Globally, AGYW are at the highest risk for HIV acquisition. In sub-Saharan Africa, AGYW are up to four times more likely to be infected with HIV than their male equivalents due to sociocultural issues including gender based violence, child marriage, and early pregnancy with subsequent low secondary school completion rates and lower socio-economic independence19. The PEPFAR DREAMS initiative aims to reduce HIV among AGYW in sub-Saharan Africa. Over 50% of new HIV infections globally occur in this region, with AGYW accounting for a third of these19.
Several randomized clinical trials have been performed among women in sub-Saharan Africa, including young women. The FEM-PrEP11 and VOICE22 trials recruited women over the age of 17 and compared oral PrEP to placebo or to placebo and TDF vaginal gel, respectively. Unfortunately, both trials showed no effect of PrEP due to poor adherence. Younger, unmarried women had poorer adherence22. A South African study (ADAPT) evaluated daily oral TDF-FTC vs two different methods of on-demand PrEP, taken only at times of sexual activity, among women 18 years or older. Daily PrEP led to increased coverage of sex events and better adherence, with no significant difference in adherence between age groups23. This is the first study showing satisfactory PrEP adherence among young women in sub-Saharan Africa. No studies have evaluated the use of PrEP in adolescent girls and while their HIV risk is similar to that of young women, adolescent girls may face additional age-related barriers to PrEP, such as intensified stigma and disapproval from parents or peers.
Male adolescents and young men who have sex with men
In the United States, 27% of new HIV diagnoses among MSM occur between the ages of 13 and 24. Of all new HIV diagnoses in this age group, 92% are gay or bisexual24, which led to the first and only published study to date assessing feasibility of daily oral PrEP in an exclusively adolescent population. Trial ATN-113 was published in September 2017. It included adolescent MSM from the ages of 15 and 17 and showed daily TDF-FTC to be well-tolerated. Drug levels were detected in more than 95% of participants in the first 12 weeks, but detectable levels decreased thereafter, correlating with a scheduled decrease in frequency of follow-up visits from monthly to quarterly7. A similar trend in adherence was observed in trial ATN-110, among MSM ages 18-2225. On-demand PrEP has demonstrated 97% efficacy in reducing HIV incidence among transgender women and MSM ages 18 or older26, and may be a future option for high risk male adolescents, but data are lacking. Other risk groups
Adolescents who inject drugs are at substantial risk of HIV infection due to shared injection equipment, disinhibited sexual behavior, and transactional sex. There has only been one randomized control trial evaluating PrEP among people who inject drugs, which demonstrated good efficacy, but did not include adolescents27.
HIV uninfected long-term sexual partners of HIV infected persons are a high risk group that can benefit from PrEP. The Partners PrEP study found the efficacy of oral PrEP in preventing HIV transmission to male and female partners to be lower in the 18-24 age group compared to those 25 years or older, possibly due to differences in adherence. However, those in the younger age group still experienced a 41-72% risk reduction9. PrEP for sero-discordant couples during adolescence is likely less common due to more transient sexual relationships during this period, one notable exception being age-discordant relationships among AGYW in sub-Saharan Africa19.
Several additional studies evaluating PrEP use in adolescent populations are underway4,28,29, including a study evaluating PrEP in pregnant and post-partum AGYW between the ages of 16 and 2430. UNICEF also is conducting a PrEP demonstration project among adolescents in Brazil, South Africa and Thailand to assess facilitators of adherence such as mHealth, peer support, self-improvement models, resilience support, and social media strategies. It is cosponsored by respective ministries of health, increasing the likelihood of generalizability31.
Safety of PrEP
TDF is generally nontoxic, but long-term use is associated with renal, endocrine, and bone toxicities32. PrEP trial sub-studies in adolescent and young MSM identified decreased bone mineral density with PrEP use. This was associated with higher parathyroid hormone levels and lower fibroblast growth factor 23 levels, but not with renal abnormalities, suggesting that adolescents may be particularly vulnerable to TDF adverse effects7,32. Tenofovir alafenamide (TAF) with FTC (trade name Descovy®) may be an alternative to TDF/FTC for PrEP, and phase 3 clinical trials are underway33. Looking ahead, an alternative long-acting injectable PrEP containing cabotegravir has been shown to be safe as a long-acting injectable PrEP in phase 2 trials34, which could also help to improve adherence.
HIV infection can be acquired with inadequate adherence to PrEP. In these situations the fluctuating serum concentrations of antiretrovirals are not sufficient to prevent acquisition of infection, but they may be present in concentrations that contribute to HIV viral mutations and subsequent antiretroviral resistance. While frequent HIV testing is done to avoid this risk, resistance mutations were seen in 25% of new HIV acquisitions in the treatment group of the Partners PrEP study9.
Adherence
Adherence is essential to the efficacy of PrEP. Data from ATN-113 suggests that adherence to oral PrEP will also be a major challenge for adolescents, and more frequent clinic visits may be the best first step to improve adherence7. Stigma and disapproval from family, partners and peers is a deterrent for many. Forgetfulness, perception of low HIV risk, concerns over side effects, potential toxicities, and cost were all reasons for poor adherence among adults in PrEP clinical trials35. Ethnographic factors in each community will present unique adherence barriers, which may be best addressed through peer navigators, support groups, and social networking36, though a multi-faceted approach for adolescents is likely necessary (Figure 1).
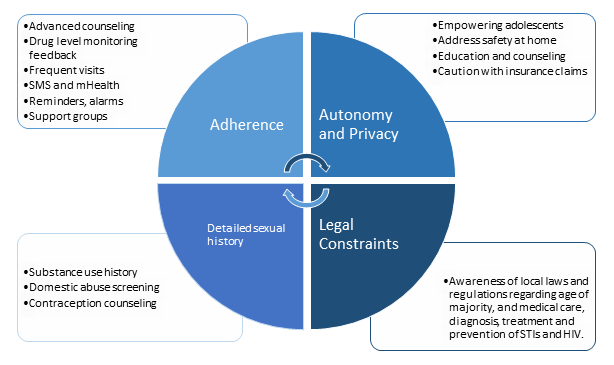
Figure 1: Barriers and facilitators to PrEP for adolescents at high risk for HIV infection
Specific approaches to supporting PrEP adherence have been evaluated in adults, although adherence facilitators may not be the same for adolescents and further studies are needed to evaluate this. Facilitators to PrEP adherence in adults include reminders, peer support, routines, advanced counseling, drug monitoring feedback and mHealth interventions37. Two specific advanced counseling methods, LifeSteps and Next-step, both involve motivational interviewing and problem solving. Through Next-step counseling interviews, used in the iPrEx Open Label Extension38,39, the most commonly reported facilitator of PrEP adherence was linking the dose to a daily routine event (86%), and the most common barrier was disruption in routine (37%)40. LifeSteps was used in an ancillary study of Partners PrEP and was credited with an 8% increase in adherence41. Drug detection feedback for use in adherence counseling has been evaluated qualitatively and was a motivational factor for some42. The use of mobile phone applications and text messages has also improved PrEP adherence in adult studies43.
Access to PrEP
While adolescents at high HIV risk generally have positive attitudes towards PrEP44, the ability to find PrEP, afford it, and maintain privacy may be challenging. PrEP uptake has been low in the United States, especially in primary care5,45. The role of prescribing antiretroviral therapy has historically and primarily resided with the Infectious Disease specialist, and there is reticence among primary care physicians to prescribe PrEP46. As of 2015, only one in three primary care physicians had even heard of PrEP47. However, providing PrEP does not require formal certification and primary care physicians are in an optimal position to reach the broadest base of high risk patients. A PrEP tool kit targeted at primary care physicians is available through the Sexuality Information and Education Council of the United States (http://www.siecus.org/index.cfm?fuseaction=page.viewPage&pageID=1555). Table 2 offers guidance for initiating PrEP in adolescents.
Table 2: Guidance for Initiating PrEP in High Risk Adolescents
| Guidance for Initiating PrEP in High Risk Adolescents |
|---|
| 1) Anticipatory guidance should be given regarding stigma. Discussions regarding disclosure of PrEP to friends, families and sexual partners are valuable, upon initiation and in follow-up. |
| 2) Adverse effects of oral TDF-based PrEP must be discussed, including a risk for nephrotoxicity and decreased bone mineral density, both being reversible with discontinuation of PrEP (5). |
| 3) Side-effects of TDF-FTC must be reviewed, including the “start-up syndrome,” which consists of nausea, mild abdominal pain or headache in the first four weeks of use and only occurs in 10% of people (5). |
| 4) STI counseling should be given, underscoring the continued risk for other STIs without condom use. |
| 5) Adolescents should be informed that their risk for new HIV infection is not eliminated, making routine follow up appointments and laboratory testing important. |
| 6) Patients who are started on PrEP require follow-up every 1-3 months for monitoring for new HIV infection, renal function, pregnancy, and adherence support. |
| 7) The need for PrEP should be re-evaluated as behaviors and sexual partners change over time. |
| 8) For adolescents with substance use disorders, counseling on safe injection practices and provision of resources for addiction treatment should be provided. |
In the United States, most health insurance companies do cover PrEP. Using parental health insurance may lead to privacy concerns for adolescents seeking PrEP through explanation of benefits letters or other health insurance paperwork. Many other adolescents and youth are uninsured, impeding access to PrEP services48. Those in rural areas may find it particularly challenging to access PrEP49. An online resource is available to help find PrEP for those without insurance (https://preplocator.org/).
In the United States, the laws pertaining to testing, treatment, and prevention of sexually transmitted infections without parental consent vary by state. Awareness of these laws and regulations are necessary for ethical provision of PrEP, as most minors cannot legally consent to PrEP alone. Emancipated minors, those with children, those in the military, and minors assessed as being “mature” may all be able to consent to PrEP, depending on state law50. In South Africa, children ages 12 and older can consent to medical treatment without parental consent if they are able to understand the risks and benefits of the medical treatment51. Each country’s regulations regarding privacy, age of consent, payer options and government funding will result in a unique landscape for the provision of PrEP to high risk adolescents.
Conclusion
The goal of preventing new HIV infections among adolescents is critical. Though there is progress, substantial gaps remain in adolescent HIV prevention research. PrEP is an available and potentially highly effective method of HIV prevention for adolescents at elevated risk, but identifying these adolescents and linking them to PrEP care may be challenging. Additionally, adherence is both essential to PrEP efficacy and particularly difficult for young persons, making strategies to improve adherence an important next step in PrEP public health interventions and research.
References
- Gray ME, Nieburg P, Dillingham R. Pediatric human immunodeficiency virus continuum of care: A concise review of evidence-based practice. Pediatr Clin North Am. 2017 Aug; 64(4): 879-91.
- Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV [homepage on the Internet]. Geneva. 2015 Sep. Available from: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1.
- Age limits and adolescents. Paediatr Child Health. 2003 Nov; 8(9): 577.
- Hosek S, Celum C, Wilson CM, et al. Preventing HIV among adolescents with oral PrEP: Observations and challenges in the United States and South Africa. J Int AIDS Soc. 2016 Oct 18; 19(7(Suppl 6)): 21107.
- Allen E, Gordon A, Krakower D, et al. HIV preexposure prophylaxis for adolescents and young adults. Curr Opin Pediatr. 2017 Aug; 29(4): 399-406.
- Bekker LG, Gill K, Wallace M. Pre-exposure prophylaxis for South African adolescents: What evidence. S Afr Med J. 2015 Nov; 105(11): 907-11.
- Hosek SG, Landovitz RJ, Kapogiannis B, et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr. 2017 Sep 5.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30; 363(27): 2587-99.
- Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2; 367(5): 399-410.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 2; 367(5): 423-34.
- Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 Aug 2; 367(5): 411-22.
- Truvada package insert [homepage on the Internet]. 2013 April 2016 [cited September 19, 2017]. Available from: http://www.gilead.com/pdf/truvada_pi.pdf.
- Hendrix CW, Chen BA, Guddera V, et al. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013; 8(1): e55013.
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3; 329(5996): 1168-74.
- Husnik MJ, Brown ER, Marzinke M, et al. Implementation of a novel adherence monitoring strategy in a phase III, blinded, placebo-controlled, HIV-1 prevention clinical trial. J Acquir Immune Defic Syndr. 2017 Nov 1; 76(3): 330-7.
- Jackson A, McGowan I. Long-acting rilpivirine for HIV prevention. Curr Opin HIV AIDS. 2015 Jul; 10(4): 253-7.
- Schlesinger E, Johengen D, Luecke E, et al. A tunable, biodegradable, thin-film polymer device as a long-acting implant delivering tenofovir alafenamide fumarate for HIV pre-exposure prophylaxis. Pharm Res. 2016 Jul; 33(7): 1649-56.
- Preexposure prophylaxis for the prevention of HIV infection in the United States - 2014 Clinical practive guideline [homepage on the Internet]. Centers for Disease Control and Prevention. 2014 [cited December 4, 2017]. Available from: https://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf.
- PEPFAR: Recommendations on the use of PrEP for all populations [homepage on the Internet]. PEPFAR Scientific Advisory Board October 14, 2015 [cited September 18, 2017]. Available from: https://www.pepfar.gov/documents/organization/250044.pdf.
- PrEP for adolescents: Successes, challenges, and opportunities [homepage on the Internet]. 2015 November 8, 2015 [cited September 19, 2017]. Available from: https://cdn.hivguidelines.org/wp-content/uploads/20160825080651/HIVG_AdolescentPrep_Meeting-report_final_02-18-20161.pdf.
- UNICEF: PrEP use among sexually active older adolescents [homepage on the Internet]. 2015 [cited September 19, 2017]. Available from: http://www.avac.org/sites/default/files/resource-files/PrEP_Use_in_Adolescents_Consultation_Report_Vancouver_Canada_July_2015.pdf.
- Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015 Feb 5; 372(6): 509-18.
- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT cape town trial): A randomised, open-label, phase 2 trial. Lancet HIV. 2017 Oct 3.
- HIV by group [homepage on the Internet]. Centers for Disease Control and Prevention. 2017 [cited December 4, 2017]. Available from: https://www.cdc.gov/hiv/group/index.html.
- Hosek SG, Rudy B, Landovitz R, et al. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017 Jan 1; 74(1): 21-9.
- Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: An observational cohort study. Lancet HIV. 2017 Sep; 4(9): e402-10.
- Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok tenofovir study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013 Jun 15; 381(9883): 2083-90.
- HPTN 082 uptake and adherence to daily oral PrEP as a primary prevention strategy for young African women: A vanguard study [homepage on the Internet]. [cited December 4, 2017]. Available from: https://www.hptn.org/research/studies/hptn082.
- Buttolph J, Inwani I, Agot K, et al. Gender-specific combination HIV prevention for youth in high-burden settings: The MP3 youth observational pilot study protocol. JMIR Res Protoc. 2017 Mar 8; 6(3): e22.
- IMPAACT 2009 (DAIDS ID 30020): Pharmacokinetics, feasibility, acceptability, and safety of oral pre-exposure prophylaxis for primary HIV prevention during pregnancy and postpartum in adolescents and young women and their infants [homepage on the Internet]. 2014 [cited September 25, 2017]. Available from: http://www.impaactnetwork.org/studies/IMPAACT2009.asp.
- UNICEF PrEP demonstration program [homepage on the Internet]. 2016 [cited September 25, 2017]. Available from: http://www.avac.org/trial/unicef-prep-demonstration-program.
- Havens PL, Stephensen CB, Van Loan MD, et al. Decline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxis. Clin Infect Dis. 2017 Feb 1; 64(3): 317-25.
- Massud I, Mitchell J, Babusis D, et al. Chemoprophylaxis with oral emtricitabine and tenofovir alafenamide combination protects macaques from rectal simian/human immunodeficiency virus infection. J Infect Dis. 2016 Oct 1; 214(7): 1058-62.
- Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): A multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017 Aug; 4(8): e331-40.
- Corneli A, Perry B, McKenna K, et al. Participants' explanations for nonadherence in the FEM-PrEP clinical trial. J Acquir Immune Defic Syndr. 2016 Apr 1; 71(4): 452-61.
- Garcia J, Colson PW, Parker C, et al. Passing the baton: Community-based ethnography to design a randomized clinical trial on the effectiveness of oral pre-exposure prophylaxis for HIV prevention among black men who have sex with men. Contemp Clin Trials. 2015 Nov; 45(Pt B): 244-51.
- Corneli A, Perry B, Agot K, et al. Facilitators of adherence to the study pill in the FEM-PrEP clinical trial. PLoS One. 2015 Apr 13; 10(4): e0125458.
- R Amico K, McMahan V, Goicochea P, et al. Supporting study product use and accuracy in self-report in the iPrEx study: Next step counseling and neutral assessment. AIDS Behav. 2012 Jul; 16(5): 1243-59.
- Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect Dis. 2014 Sep; 14(9): 820-9.
- Chianese C, Amico KR, Mayer K, et al. Integrated next step counseling for sexual health promotion and medication adherence for individuals using pre-exposure prophylaxis. AIDS Research and Human Retroviruses. October 2014; 30(S1): A159-A159.
- Psaros C, Haberer JE, Katabira E, et al. An intervention to support HIV preexposure prophylaxis adherence in HIV-serodiscordant couples in Uganda. J Acquir Immune Defic Syndr. 2014 Aug 15; 66(5): 522-9.
- Koester KA, Liu A, Eden C, et al. Acceptability of drug detection monitoring among participants in an open-label pre-exposure prophylaxis study. AIDS Care. 2015; 27(10): 1199-204.
- Sullivan PS, Driggers R, Stekler JD, et al. Usability and acceptability of a mobile comprehensive HIV prevention app for men who have sex with men: A pilot study. JMIR Mhealth Uhealth. 2017 Mar 9; 5(3): e26.
- Koechlin FM, Fonner VA, Dalglish SL, et al. Values and preferences on the use of oral pre-exposure prophylaxis (PrEP) for HIV prevention among multiple populations: A systematic review of the literature. AIDS Behav. 2017 May; 21(5): 1325-35.
- Walsh JL, Petroll AE. Factors related to pre-exposure prophylaxis prescription by U.S. primary care physicians. Am J Prev Med. 2017 Jun; 52(6): e165-72.
- Calabrese SK, Magnus M, Mayer KH, et al. Putting PrEP into practice: Lessons learned from early-adopting U.S. providers' firsthand experiences providing HIV pre-exposure prophylaxis and associated care. PLoS One. 2016 Jun 15; 11(6): e0157324.
- CDC: Daily pill can prevent HIV [homepage on the Internet]. 2015 [cited September 28, 2017]. Available from: https://www.cdc.gov/vitalsigns/hivprep/index.html.
- Patel RR, Mena L, Nunn A, et al. Impact of insurance coverage on utilization of pre-exposure prophylaxis for HIV prevention. PLoS One. 2017 May 30; 12(5): e0178737.
- Hubach RD, Currin JM, Sanders CA, et al. Barriers to access and adoption of pre-exposure prophylaxis for the prevention of HIV among men who have sex with men (MSM) in a relatively rural state. AIDS Educ Prev. 2017 Aug; 29(4): 315-29.
- Culp L, Caucci L. State adolescent consent laws and implications for HIV pre-exposure prophylaxis. Am J Prev Med. 2013 Jan; 44(1 Suppl 2): S119-24.
- McQuoid-Mason D. Provisions for consent by children to medical treatment and surgical operations, and duties to report child and aged persons abuse: 1 April 2010. SAMJ: South African Medical Journal. 2010; 100(10): 646-8.
